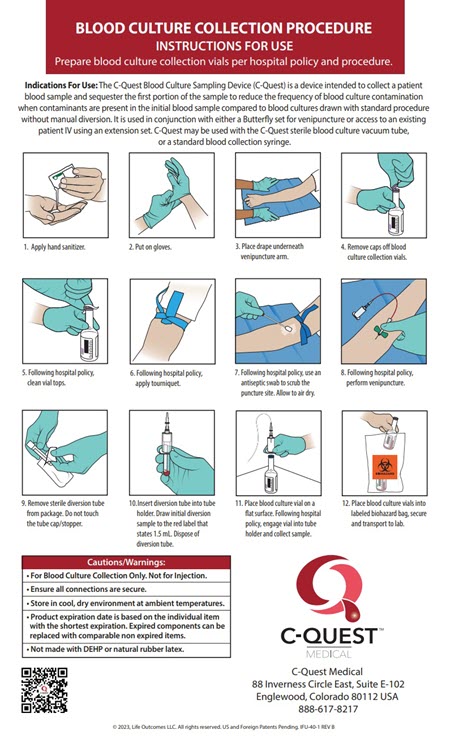
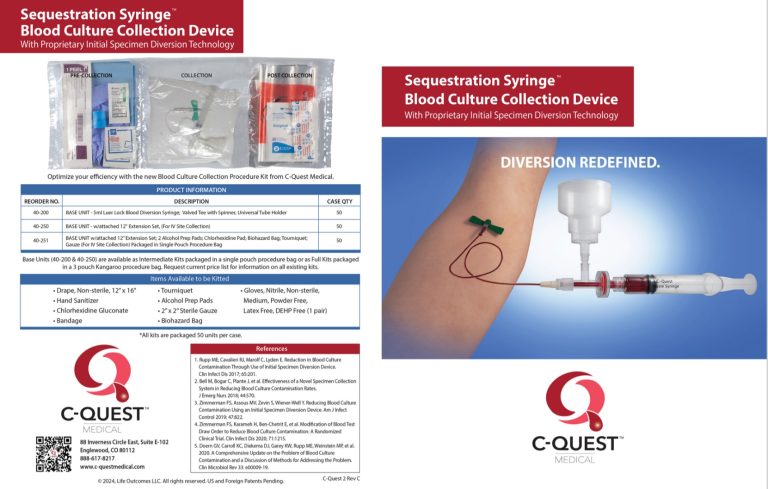
C-Quest Medical
Blood Culture Collection Devices with Initial Specimen Diversion Technology
Blood culture tests have long been the standard of care to detect serious blood stream infections. However, false positive blood-culture test results, which generally stem from contaminated skin samples, can reach as high as 50%. This unsettling statistic is cause for significant clinical and cost concerns for healthcare professionals worldwide.
Objectives
- Introduce C-Quest Medical Diversion Devices to blood culture collection healthcare professionals.
- Position C-Quest Medical Diversion Devices as the innovative, easy-to-use, and economical alternative to commercially available options.
Strategy
- Develop an integrated Marketing Communications Launch Campaign with Exhibit Graphics, Collateral, Instructions for Use, and Website (https://www.c-questmedical.com).
- Create a relevant and distinctive Brand Logo that implies blood diversion.
- Create a Brand Identity Statement that communicates company goals.
- Emphasize product simplicity and ease-of-use in all communications.
- Highlight cost savings.
Target Audience
- Emergency Room Nurses
- Phlebotomists
- Clinical Laboratory Technicians
- Infection Control Teams
Numerous factors can cause blood culture contamination. However, many clinical and scientific studies conclude the use of “initial specimen diversion devices” during blood collection can be effective in reducing the rate of blood culture contamination.
Until now, the high cost and complexity of available initial blood specimen diversion devices have hindered their acceptance.